Background: Increased thrombin generation is an interesting candidate as a potential indicator of hypercoagulability and thrombosis risk. Increased thrombin generation using the calibrated automated thrombogram (CAT) has been reported in antithrombin deficiency but the CAT does not reflect protein C deficiency without the addition of thrombomodulin (TM). TM activates protein C (PC) in the CAT and reduces thrombin generation.
Objective: The objective of this study was to determine the capacity of the TM-augmented CAT to detect defects in various protein C system components; the lupus anticoagulant was also investigated as a cause of decreased protein C activation.
Methods: The CAT-TM was performed with reagents and methods per the manufacturer's instructions using 5 pM tissue factor and TM (Stago). Other assays included: PC (chromogenic), free protein S (PS, LIA), activated protein C resistance (aPTT-based clotting assay), factor V Leiden (FVL PCR) and lupus anticoagulant (LA, dRVVT and Staclot LA, Stago). Platelet poor plasma samples were obtained from the biobank of a consented prospective inceptional cohort study of thrombosis and thrombophilia or a positive family history of either (ThromboPICS #05-0339); samples from healthy controls with no personal or family history of thrombosis or thrombophilia were collected on a consented protocol (#09-0816). Samples from participants on therapeutic inhibitors of factor Xa or thrombin were treated with appropriate neutralizers; no sample was tested on warfarin. Clinical data included gender, age (< 18 versus ≥ 18 years), history of thrombosis, timing of sample from most recent thrombosis (acute < 14 days; subacute 14-90 days; or chronic > 90 days) and use of anticoagulants at the time of the blood draw. Tests for violations of normality were negative. Therefore, results of cohorts versus controls were compared with independent sample t-test and subgroup analyses relative to control used One-Way ANOVA. Post-hoc comparisons of each subgroup to the controls were collected for multiple comparisons using the Bonferroni correction.
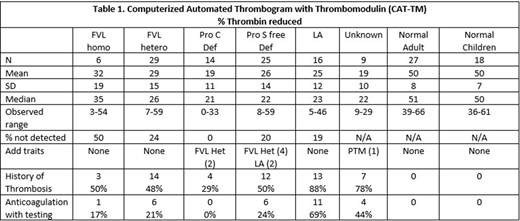
Results: Ninety-nine cases and 45 normal controls were studied. Overall half of the cases had a history of thrombosis and 66% of those with thrombosis were on no anticoagulation at the time of testing. Table 1 displays results of the CAT-TM in the 2 control and 6 study groups. Control adults and children showed a mean 50% thrombin reduction in thrombin generation with CAT-TM; overall, persons with protein C system defects showed approximately 25% reduction, with the least reduction of thrombin generation in the cohort of PC deficiency at 19%. Sensitivity of the CAT-TM was 100% for PC deficiency, 81% for LA positivity, 80% for PS deficiency and 72% for FVL positivity with no difference in degree of thrombin reduction between hetero- and homozygous FVL. In addition, we identified 14 individuals with CAT-TM results similar to protein C system defects but with confirmed normal PC, PS, FVL and LA tests. Although 9 of these unknowns had a personal (7) or family (2) history of thrombosis, five came from the healthy controls. The false positive results in 3 adult and 2 pediatric controls conferred a CAT-TM test specificity of 89%. Furthermore, analyses showed no differences in CAT-TM results related to gender, age group, history of thrombosis, age of clot at blood draw (acute, subacute or chronic) or use of anticoagulation at the time of blood draw.
Conclusions: The CAT-TM is a useful screening test for defects in the protein C system, including LA, although this test could not discriminate between heterozygous and homozygous FVL. The etiology of positive CAT-TM in normal individuals or individuals with a personal or family history of thrombosis without identified PC system defects is currently unknown and under ongoing investigation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal